 Springbrook Rescue
Springbrook Rescue
 Springbrook Rescue
Springbrook Rescue
| Home | The Vision | Springbrook — A Natural Wonder | The Springbrook Rescue Project | Support the Project | About ARCS |
|
The Science | |||
| |||
|
Science Conceptual Models
Ecological Conceptual Models Ecological conceptual models simply and transparently indicate our current level of understanding of how ecological systems work (including the rainforest ecosystems at Springbrook). We have selected the following ecological conceptual model at the broadest level to most usefully represent our understanding of ecosystem structure and function as it relates to ecological restoration. It allows us to judge if and when interventions are required, to predict responses to any interventions planned and devise the best indicators by which to measure progress in reaching objectives. The model is based on that of Worm and Duffy (2003) and addresses drivers and response variables in ecosystem dynamics that ultimately affect ecosystem stability (resistance/resilience). It allows consideration of the impact of drivers of change on system processes and structural attributes of resistance and resilience to be considered at multiple levels including individuals, populations, species, communities, ecosystems and landscapes. The model facilitates nested models at finer scales. 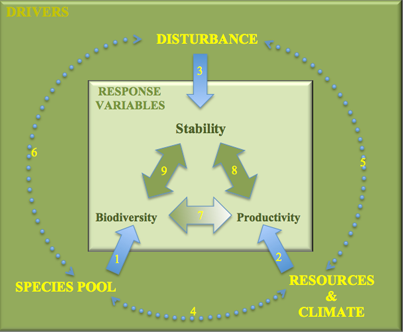
The key elements of the model (and assumptions we have adopted) are described here: THE DRIVERS Species Pool Resources and Climate Resource availability is affected by climate and processes affecting soil-water infiltration, retention, and release to plant roots, indirectly affecting nutrient availability. Disturbance We recognise that both the external “drivers” and “response” variables can act as either external or internal drivers (proximal/distal) with feedback interactions between each of them resulting in structural, functional and interaction (feedback) shifts. THE RESPONSE VARIABLES Biodiversity Taxon-based measures Richness (via functional traits) influences energy and nutrient fluxes (including carbon)(4) through increased facilitation and niche complementarity at high species richness Composition (and evenness) may affect productivity (7) through functionally dominant species that may represent a small number of species out of the total regional or local species pool. Low taxon-based diversity may reflect species pool effects, resource-climate limitation, high disturbance or high productivity and biotic interaction (competition, predation, herbivory). Trait-based measures can help distinguish between these external and internal drivers. Trait-based measures: Morphological diversity — (e.g. size diversity) can reflect food web energy fluxes, trophic structure and niche segregation Functional diversity — the number and dominance of life-history strategies within a community reflecting adaptation to natural disturbance Functional diversity can indicate not only species numbers and dominance but also their functional role in an assemblage of species. Loss of functional diversity results in altered species interactions and ecosystem function, and systems resilience. Biological traits confer resistance or resilience to stress or disturbance at either the species or assemblage level. Trait-based approaches assume that habitat (resources-climate) selects for characteristic life history traits via natural selection (Southwood 1977) over evolutionary timeframes and biogeographic scales or as a consequence of human disturbance and habitat alteration at finer scales through a balance between colonisation-extinction processes. Consequently local species pools in isolated or fragmented environments can reveal “nestedness” within the broader regional species pool through loss of sensitive species. Phylogenetic diversity — the taxonomic relatedness amongst species assemblages based on phylogenies reflecting long-term, conserved evolutionary adaptations potentially vulnerable to human impacts Taxonomic distinctness — the relatedness of species within an area or sample reflecting taxonomic breadth (a simpler metric based on hierarchical Linnaean classifications) Phylogenetic diversity (and related taxonomic distinctness index) reflects evolutionary relationships amongst species within an assemblage related to long-term evolutionary adaptations and is likely to be sensitive to human impacts rather than habitat differences. Loss of phylogenetic diversity reflects loss of evolutionary history and future options. Ecological restoration must consider taxonomic, functional and phylogenetic diversity in order to restore and maintain species, functional and evolutionary processes. Productivity Increasing resource supply increases potential productivity to which local diversity adjusts (2, 7) Stability Stability encompasses the concepts of resilience and resistance to disturbance or stress. Resilience is the capacity to re-establish structure and function after disturbance. Resistance is the capacity to maintain integrity of structure and function under stress. Diversity increases stability (9) because of a greater range of adaptive functional traits within the species pool that keep productivity stable under a range of resource-climate conditions. Stability of the environment adjusts local richness within a species pool (9). Diversity within a community can stabilize/adjust the abiotic environment by ameliorating fluctuations in resource-climate conditions (water availability, ambient temperature, light levels) to influence disturbance regimes, thence diversity. This is one important functional attribute of refugia for ancient lineages of plants or animals with highly conserved traits. References Chapin III, F.S., Torn, M.S. and Tateno. M. (1996a) Principles of ecosystem sustainability. The American Naturalist 148, 1016–1037. DeAngelis, D.L. and Post, W.M. (1991) Positive feedback and ecosystem organization. Pp. 155–178 in M. Higashi and T.P. Burns, Eds. Theoretical Studies of Ecosystems: The Network Perspective. Cambridge University Press, Cambridge. DeAngelis, D.L., Post, W.M. and Travis, C.C. (1986) Positive Feedback in Natural Systems. Springer, Berlin. Drake, J.A. (1990). The mechanics of community assembly and succession. Journal of Theoretical Biology 147, 213-233. Law, R. and Morton, R.D. (1993). Alternative permanent states of ecological communities. Ecology 74(5), 1347-1361. Prober, S.M., Thiele, K.R., Rundel, P.W., Yates, C.J., Berry, S.L., Byrne, M., Christidis, L., Gosper, C.R., Brierson, P.F., Lemson, K., Lyons, t., Macfarlane, C., O’Connor, M.H., Scott, J.K., Standish, R.J., Stock, W.D., van Etten, E.J.B., Wardell-Johnson, G.W. and Watson, A. (2011). Climatic Change. DOI 10.1007/s10584-011-0092-y. Southwood, T.R.E. (1977). Habitat, the templet for ecological strategies. Journal of Animal Ecology 46, 337-365. Vellend, M., Cornwell, W.K., Magnuson-Ford, K. and Mooers, A.O. (2011). Measuring phylogenetic biodiversity. In Biological Diversity: Frontiers in Measurement and Assessment. Eds A.E. Magurran and B.J. McGill. Oxford University Press. Warwick, R.M. and Clarke, K.R. (1995). New ‘biodiversity’ measures reveal a decrease in taxonomic distinctness with increasing stress. Marine Ecology Progress Series 129, 301-305. Worm, B. and Duffy, J.E. (2003) Biodiversity, productivity and stability in real food webs. Trends in Ecology and Evolution 18, 628–632. |
|||